
Biological Safety Testing Products And Services Market Size, Share & Trends Analysis Report By Product (Reagents & Kits, Services), By Application (Vaccines & Therapeutics, Gene Therapy), By Test Type, By Region, And Segment Forecasts, 2023 - 2030
- Report ID: GVR-1-68038-140-5
- Number of Pages: 236
- Format: Electronic (PDF)
- Historical Range: 2018 - 2021
- Industry:Healthcare
Report Overview
The globalbiological safety testing products and services market sizewas valued atUSD 4.12 billion in 2022and is expected to grow at a compound annual growth rate (CAGR) of 10.7% from 2023 to 2030. The growing prevalence of target diseases and rising production of next-generation biologics by various biotechnology and pharmaceutical organizations are anticipated to boost the biological safety testing products & services market. For instance, in September 2022, Novartis announced its investment of USD 300 million in next-generation biotherapeutics, bolstering the capacity for the early technical expansion of biologics.
The growing demand for biologics has led to an extraordinary increase in the number of biopharmaceutical companies. The rising competition to produce highly effective therapeutic drugs on a large scale has enabled manufacturers to focus on enhancing aspects of industrial processes, including cost-effectiveness and productivity. For instance, in July 2023, Biocon Biologics, an Indian-based organization, announced the launch of the rheumatoid arthritis drug Humira, a biosimilar version of AbbVie in the U.S. at a lower price that would be easily available across the region. Several organizations are thus executing better manufacturing practices involving thoroughbiological testingat various levels of the production cycle with easy accessibility, thereby fueling the growth of the market.
The growing number of government initiatives to stimulate biological safety testing products and services is anticipated to propel overall growth over the forecast period. For instance, in June 2022, Pfizer and BioNTech announced their collaboration with the Government of the U.S. to offer additional vaccine supply to control the spread of the COVID-19 virus. Thus, government and private organizations are anticipated to enhance underlying biological safety practices in response to the elevated occurrence of microbial contamination and bioburden during the manufacturing of pharmaceuticals and biologics.
Moreover, the presence of regulatory authorities to enforce significant safety standards is anticipated to boost the adoption of testing tools. For instance, in June 2023, the FDA introduced a new guideline that would encourage biosimilar developers to adopt a more cost-effective approach to testing. The FDA guideline, called "BioRationality," aims to promote a more scientifically rational and efficient approach to the development and approval of biosimilars. The new guideline suggests alternative testing methodologies to streamline the development process and reduce costs. Stringent guidelines and recommendations issued by these authorities increase the incorporation of these tools by quality assurance technicians, thus fueling market growth.
Biological safety testing services play a crucial role in verifying the absence of bacterial contaminants and ensuring the safety of vaccines and biopharmaceuticals. These services encompass a range of assessments, including bioburden testing, toxicology testing, and analytical testing. Parameters such as accuracy, linearity, range, and specificity are evaluated to assess the quality of products offered by companies in this field.
Product Insights
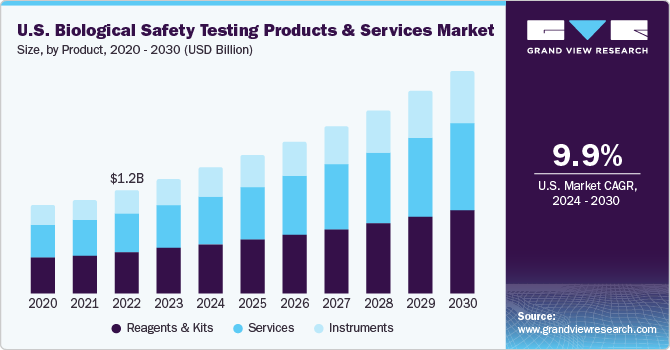
Based on product, reagents and kits accounted for the largest market share of 40.27% in 2022. Reagents are a major component in biological safety testing and are hence extensively used in research and clinical laboratories. These reagents areantibiotics, attachments & matrix factors, biological buffers, freezing & dissociation reagents, and miscellaneous reagents. Rapid advancements and modifications in the formulation of reagents and kits are expected to increase their adoption by laboratory technicians, especially in toxicology assessment. Additionally, the increasing demand for high-throughput testing has contributed to the popularity of reagents and kits. These products enable efficient and rapid testing of large sample volumes, making them suitable for screening purposes and high-volume production environments.
The instruments segment is expected to grow at fastest growth rate during the forecast period due to rising demand for instruments in biological safety testing laboratories. Additionally, the increasing regulatory pressure on biopharmaceutical companies to adhere to safety guidelines drives the demand for instruments. They play a crucial role in conducting biological assays, including toxicology and bioburden tests. Moreover, the market is witnessing a rising demand for specialized biological safety testing, such as genetic testing,cell-based assays, and flow cytometry. These testing methods require specific instruments to carry out the procedures accurately and efficiently. The increasing adoption of personalized medicine and targeted therapies also contributes to the demand for instruments that are suitable for specialized testing needs.
Application Insights
The vaccine and therapeutics segment held the largest share, based on application, in 2022 with a market share of 23.58% and is anticipated to grow at fastest growth rate over the forecast period. The segment’s dominance is attributed to the presence of clearly defined guidelines ensuring the safety of developed vaccines with unaltered therapeutic value and reduced toxicity. In January 2023, BioNTech SE announced their collaboration Memorandum of Understanding with the UK Government, which would help patients by fast-tracking clinical trials for mRNA personalized immunotherapies. The collaboration is anticipated to focus on three things, includingcancer immunotherapies, infectious disease, and vaccines, thus, strengthening their presence in the UK. Thus, the growth of this segment is expected to be fueled by the issuance of various guidelines and recommendations by regulatory authorities, such as the U.S. FDA, regarding the characterization and qualification of materials utilized in the production of vaccines for infectious disease indications.
Additionally, thegene therapysegment is anticipated to experience substantial growth during the forecast period due to the heightened risk of contamination with residual DNA. The presence of residual DNA is considered a potential risk to the final product due to its high potential for infectivity. Furthermore, mAbs play a vital role in the identification and characterization of critical quality attributes (CQAs) of biopharmaceuticals. These CQAs are specific characteristics that define the product’s safety, purity, and potency. Monoclonal antibodies are used in assays to assess parameters like product-related impurities, degradation products, and host cell proteins, ensuring that the biopharmaceuticals meet the required quality standards. Thus, the demand for monoclonal antibodies (mAbs) in the biological safety testing product and services market is experiencing a significant increase.
Test Type Insights
Endotoxin tests dominated the biological safety testing product & services market in terms of revenue in 2022, with a market share of 21.96%. High revenue share can be attributed to the rising usage of these tests in various areas, such as the manufacturing and producing drugs to reduce the threat of endotoxins. In August 2022, Lonza announced the launch of Nebula Multimode Reader, the first qualified reader for use in the company’s recombinant endotoxin, turbidimetric, and chromogenic detection methods. The accessibility of several types of endotoxin tests, such as turbidimetric method, gel clot endotoxin testing, and USP chromogenic endotoxin testing, which are designed for different requirements, is likely to boost segment growth. In addition, endotoxin testing plays a crucial role in ensuring patient safety by preventing the release of pyrogen-contaminated products into the market. Controlling endotoxin levels in pharmaceuticals and medical devices helps minimize the risk of adverse reactions and infections in patients. Manufacturers prioritize patient safety by implementing rigorous endotoxin testing protocols, thereby driving the demand for these tests.
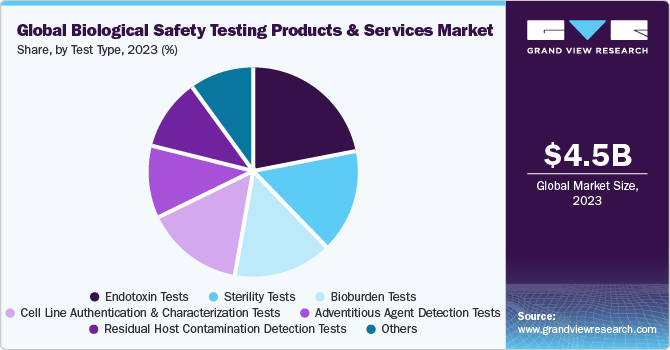
Thebioburden testssegment is expected to grow at fastest growth rate over the forecast period. This can be attributed to the high adoption of these tests to determine the bioburden limit in a wide range of biologics and medical devices. In April 2023, STEMart, a U.S.-based provider, announced the launch of bioburden and sterility testing for medical devices under the regulation of the ISO 11731 technique. Moreover, integrating cutting-edge colorimetric methods and computation has facilitated the rapid generation of results and accurate bioburden quantification. In addition, Bioburden testing helps identify and quantify the microbial load present in these products. By establishing acceptable limits for microbial contamination, bioburden testing assists in mitigating the risk of infection and adverse reactions in patients. Rapid advancements in this segment are further expected to aid in segment growth.
Regional Insights
North America held the largest share of the global market in 2022, with a market share of 35.27%. The significant market share can be attributed to substantial investments in biotechnology, growing adoption in cancer research, and the advancement of novel biologics, vaccines, and drugs. Additionally, increasing research and development (R&D) investments by companies is a key factor driving growth. Furthermore, the market is boosted by major market players undertaking extensive expansion strategies aided by the rising prevalence of chronic diseases in this region is expected to drive the adoption of advanced technologies by researchers and healthcare professionals, thereby expanding the market growth.
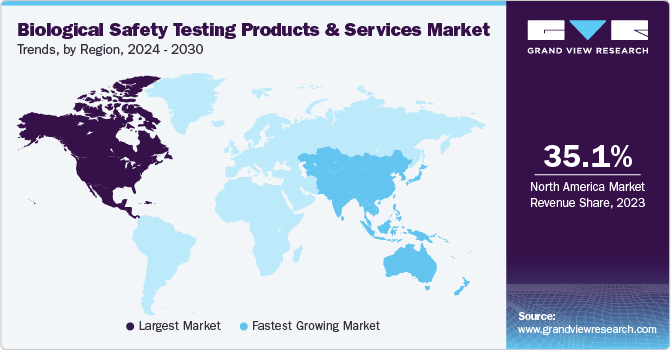
Moreover, North America has well-established regulatory bodies, such as FDA, which sets stringent guidelines and requirements for biological safety testing. Compliance with these regulations makes it crucial for manufacturers to ensure the safety and quality of their products. Such stringent regulatory environment encourages the adoption of comprehensive biological safety testing, driving market dominance.
The Asia Pacific market is anticipated to grow at a significant CAGR over the forecast period. Factors such as the growth in healthcare spending and rising awareness of the advantages associated with these products are anticipated to contribute to market growth. Additionally, the presence of organizations such as the Asia Pacific Biosafety Association, which plays a pivotal role in providing training on biosafety principles and practices to professionals throughout the region, is further expanding growth opportunities in the region.
Key Companies & Market Share Insights
Market leaders are involved in extensive R&D for manufacturing cost-efficient and technologically advanced testing products. Several strategies, such as mergers & acquisitions, undertaken by these organizations to expand their market presence are anticipated to create significant growth opportunities over the forecast period. For instance, in January 2023, Charles River Laboratories, Inc. acquired SAMDI Tech, Inc, which is a provider of high-throughput screening (HTS) solutions for several drug discovery research. Thus, intensifying its capacity to offer drug discovery and development services. Some of the key players operating in the global biological safety testing products and services market include:
Charles River Laboratories
BSL Bioservice
Merck KGaA (MilliporeSigma)
Samsung Biologics
Sartorius AG
Eurofins Scientific
SGS Société Générale de Surveillance SA
Thermo Fisher Scientific Inc.
BIOMÉRIEUX
Lonza
Biological Safety Testing Products And Services Market Report Scope
Report Attribute |
Details |
Market size value in 2023 |
USD 4.57 billion |
Revenue forecast in 2030 |
USD 9.31 billion |
Growth rate |
CAGR of 10.7% from 2023 to 2030 |
Base year for estimation |
2022 |
Historical data |
2018 - 2021 |
Forecast period |
2023 - 2030 |
Report updated |
September 2023 |
Quantitative units |
收入在十亿美元/百万,从2023年的复合年增长率to 2030 |
Report coverage |
Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
Segments covered |
Product, application, test type, region |
Regional scope |
北美;欧洲;亚太地区;拉丁美洲; MEA |
Country scope |
U.S.; Canada; UK; Germany; France; Italy; Spain; Norway; Sweden; Denmark; Japan; China; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
Key companies profiled |
Charles River Laboratories; BSL Bioservice, Merck KGaA (MilliporeSigma); Samsung Biologics; Sartorius AG; Eurofins Scientific; SGS Société Générale de Surveillance SA; Thermo Fisher Scientific Inc.; BIOMÉRIEUX; Lonza |
Customization scope |
Free report customization (equivalent up to 8 analysts’ working days) with purchase. Addition or alteration to country, regional & segment scope |
Pricing and purchase options |
Avail customized purchase options to meet your exact research needs.Explore purchase options |
GlobalBiological Safety Testing Products And Services Market ReportSegmentation
这份报告预测收入增长并提供an analysis of the latest trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global biological safety testing products and services market report based on product, application, test type, and region:
Product Outlook (Revenue, USD Million, 2018 - 2030)
Reagents & Kits
Instruments
Services
Application Outlook (Revenue, USD Million, 2018 - 2030)
Vaccines & Therapeutics
Vaccines
Monoclonal Antibodies
Recombinant Protein
Blood & Blood-based Products
Gene Therapy
Tissue & Tissue-based Products
Stem Cell
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
Endotoxin Tests
Sterility Tests
Cell Line Authentication & Characterization Tests
Bioburden Tests
Adventitious Agent Detection Tests
Residual Host Contamination Detection Tests
Others
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
拉丁美洲
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
Frequently Asked Questions About This Report
b.The global biological safety testing products and services market size was estimated at USD 4.12 billion in 2022 and is expected to reach USD 4.57 billion in 2023.
b.The global biological safety testing products and services market is expected to grow at a compound annual growth rate of 10.7% from 2023 to 2030 to reach USD 9.31 billion by 2030.
b.North America dominated the biological safety testing products and services market with a share of 35.27% in 2022. This is attributable to the presence of prominent market players undertaking extensive expansion strategies.
b.Some key players operating in the biological safety testing products & services market include Charles River Laboratories International, Inc.; BSL Bioservice Scientific Laboratories GmbH; Lonza Group AG; Milliporesigma; Sartorius Stedim BioOutsource Limited; and Samsung BioLogics.
b.推动生物安全的关键因素testing products and services market growth include an increase in the production of new-generation biologics by major pharmaceutical and biotechnology companies and the global prevalence of target diseases.
We are committed towards customer satisfaction, and quality service.
"The quality of research they have done for us has been excellent."






